STERILISATION PROTOCOL
A key document for the correct implementation of the sterilisation protocol to protect the health of operators and patients.

Collection
The sterilisation protocol begins with collecting instruments and materials. Potential operator exposure to biological agents begins with collection of the (potentially contaminated) materials used, which must be carried in closed carts specially used for this operation. The operators appointed to carry out receiving and washing operations may handle the instruments, wearing appropriate Personal Protective Equipment.
DISINFECTION
Disinfection can be carried out manually or by means of thermodisinfectors. In manual disinfection, the choice of disinfectant formulations must take into account their effectiveness against biological risk agents and compatibility with the materials to be treated. In automatic disinfection the material, placed inside the equipment, follows a specific disinfection program.
washing
The washing phase is essential to ensure correct sterilisation in the autoclave and allows organic and inorganic residues to be removed from the instruments. Washing can be done manually or mechanically through the use of ultrasonic tanks, if compatible with the materials to be treated.
Rinsing
The manual cleaning procedure must be followed by rinsing, carried out by showering the material first with running water and then with demineralised water, to remove any residual detergent left on the instruments.
Drying
After rinsing, the material is dried with paper or lint-free fabric cloths. During this stage, it is important to use suitable PPE to avoid accidental injuries.
INSPECTION AND MAINTENANCE
This is the phase preceding packaging: the materials must be carefully checked in all their parts, to ensure their efficiency and integrity, and ensure their suitability for surgical purposes. All materials with damaged parts (breaks, etc.) or rusting must be repaired or treated with specific products, receiving maintenance and lubrication as required.
PACKAGING
In the packaging phase, sterilisation pouches are used to create a physical barrier that preserves sterility until the instrument is used again. Correct packaging is essential to create and maintain a physical barrier between the instruments and the outside world, guaranteeing sterility over time.
STERILISATION
Sterilisation makes the instruments usable again. It is the stage of the reconditioning process in which any residual microorganisms not removed after washing and disinfection are rendered inactive.
TRACEABILITY
Traceability makes it possible to uniquely identify key data relating to the load and its processing, using a label to be subsequently associated with the patient's medical record.
STORAGE
Storage guarantees that instrument sterility is maintained; the FIFO system (first-in first-out) is always used. It is essential to keep sterilised instruments in their pouches, in clean storage places away from sources of heat and light.
Tethys H10 Plus
4 phases in one simple passage
Tethys H10 Plus makes the reconditioning process even simpler and more functional. Decontamination, washing, thermal disinfection and drying are all performed in a single, rapid, automatic process.
One cycle that eliminates all risks for the operator and efficiently completes the four phases that precede instruments’ packaging and sterilization.


Thermal Sealer | Millseal+ Evo
The automatic thermal sealer that shortens packaging times.
Millseal+ Evo stands out on account of its compact linear design, outstanding user-friendliness and fully automatic preparation control. After setting the length and the number of pouches you wish to prepare, just press the relative “Programme” key to start the automatic procedure.

Autoclave | SUPREME
With its unrivalled low consumption, Supreme is the new market benchmark.
We developed the first closed-loop sterilizer that uses mains through a specific internal filtration system that recycles water to be reused in the following cycles. This performance eliminates waste, reduces costs and ensures a significant increase in the efficiency of any modern dentistry surgery.
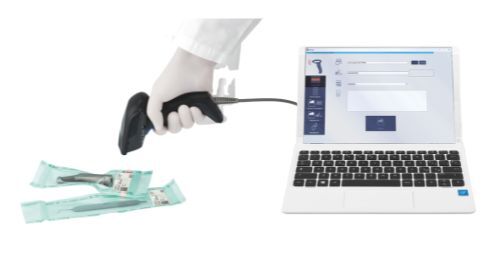
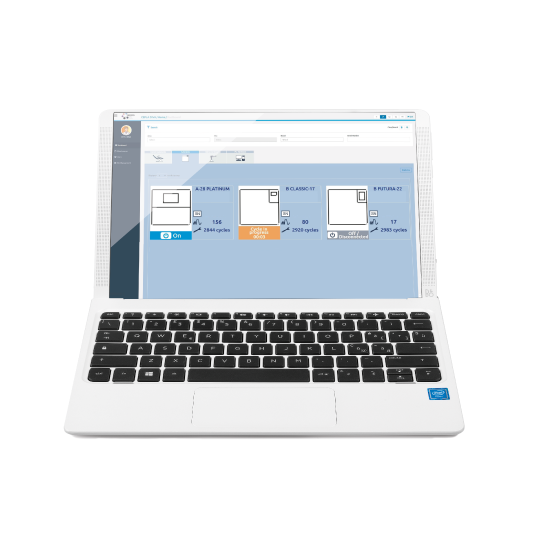
Traceability software
MyTrace is Cefla’s traceability software supplied with Supreme. By using this programme, each set of sterilized instruments can be associated to the patient through a bar code. This essential software completes the sterilization process and provides legal protection to dentists.